Covalent Chemistry
Nonmetallic elements involve hydrogen and the upper right-hand side of the p block. Covalent bonding is feature of the elements and of the compounds they create with other nonmetals. The bonding possibilities rely on the atoms' electron configurations. Hydrogen is unique and usually can create only one covalent bond. Boron is also not general as compounds such as BF3 have an incomplete octet. Electron deficiency leads to the formation of several unusual compounds, particularly hydrides (see also Topic C7). The increasing number of valence electrons among groups 14 and 18 has two feasible consequences. In simple molecules following the octet rule the valency falls along with group number (example in CH4, NH3, H2O and HF, and in associated compounds in which H is replaced by a halogen or an organic radical). Alternatively, if the number of valence electrons included in bonding is not limited, afterwards a wider range of valencies becomes possible from group 15 onwards. This is easily get in combination along with the highly electronegative elements O and F, and the resultant compounds are best classified through the oxidation state of the atom concerned. So the maximum possible oxidation state raises from +5 in group 15 to +8 in group 18. The +5 state is display in all periods (example NO3- , PF5) but higher oxidation states in later groups need octet expansion and take place only from period 3 onwards (example SF6 and CIO4- in group 18 only xenon can do this, example XeO4).
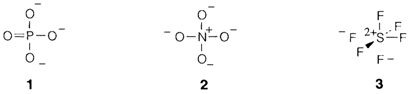
Octet hypervalence or expansion is frequently attributed to the involvement of d orbitals in similar principal quantum shell (example 3d in period 3;). So six octahedrally directed bonds as in SF6 could be created with sp3d2 hybrid orbitals. In a identical way the multiple bonding usually drawn in species like PO3-4(1) is often explained as dπ-pπ bonding. These models definitely overestimate the contribution of d orbitals. It is all the time possible to draw valence structures with no octet expansion presented that nonzero formal charges are allowed. For instance, the orthonitrate ion NO3-4is drawn with no double bonds (2), and PO3-4 could be like wise represented. One of several equivalent valence structures for SF6 in which sulfur has only eight valence-shell electrons is displayed in 3. Three-center four-electron bonding models express the same ideas. Such type of models are also oversimplified. It is usually believed that d orbitals do play some important role in octet expansion, but that two other issues are at least as significant: the larger size of elements in lower periods, that permits higher coordination numbers and their lower electronegativity, that contains positive formal charge more simply.
Other very significant distinction among period 2 elements and others is the ready formation of multiple bonds through C, N and O . several of the compounds of these elements contain structures and stoichiometries not repeated in lower periods (example oxides of nitrogen;).
A number of of these trends are exemplified through the selection of molecules and complex ions in Table 1. They have been categorized through (i) the sum of valence electrons (VE), and (ii) the steric number of the central atom (SN), that is calculated through add up the number of lone-pairs to the number of bonded atoms and employed for interpreting molecular geometries within the VSEPR model. The species listed in Table 1 demonstrate the wide range of isoelectronic relationships that present among the compounds created by elements in dissimilar groups and periods.
Species with SN=4 are found during the p block, but ones along with lower steric numbers and/or multiple bonding are general only in period 2. Within the analogous compounds with heavier elements the coordination and steric numbers are frequently raised by polymerization (compare SiO2 and CO2, [SIO2-3] and CO2-3) or through a change of stoichiometry (example. SIO4-4). Species with steric numbers higher than four need octet expansion and are not found within period 2. Several of thespecies listed in Table 1 are considered to dealing with the suitable elements.