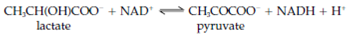Enzyme assays
The amount of enzyme protein present can be determined assayed in terms of the catalytic effect it generates, which is the conversion of substrate to product. In categorize to assay monitor the activity of an enzyme the whole equation of the reaction being catalyzed must be known and an analytical procedure must be available for determining either the disappearance of substrate or the appearance of product. Additionally, one must take into account whether the enzyme needs any cofactors and the pH and temperature at that the enzyme is optimally active. For mammalian enzymes this is commonly in the range of 25–37oC. At last, it is essential in which the rate of the reaction being assayed is a measure of the enzyme activity present and is not limited through an insufficient supply of substrate. Thus, very high substrate concentrations are commonly needed so that the initial reaction rate that is determined experimentally is proportional to the enzyme concentration.
An enzyme is very conveniently assayed through measuring the rate of disappearance of substrate or the rate of appearance of product. If the substrate (or product) absorbs light at a specific wavelength then modification in the concentration of these molecules can be measured through following the change of absorbance at this wavelength using a spectrophotometer. If the substrate (or product) fiuoresces, then changes in the concentration can be measured through following the change in fiuorescence using a fiuorimeter. While absorbance is proportional to concentration, the rate of modification in absorbance or fiuorescence is proportional to the rate of enzyme activity in moles of substrate used (or product formed) per unit time.
Two of the most general molecules used for absorbance measurement in enzyme assays are the coenzymes decrease NADH (nicotinamide adenine dinucleotide) and reduced nicotinamide adenine dinucleotide phosphate (NADPH) that each absorb in the UV (ultraviolet) region at 340 nm. Therefore, if NADPH or NADH is produced during the course of the reaction there will be a corresponding raise in absorbance at 340 nm, whilst if the reaction includes the oxidation of NADPH or NADH to NADP or NAD, respectively, there will be a corresponding reduction in absorbance, while these oxidized forms do not absorb at 340 nm. One instance is that the activity of lactate dehydrogenase with lactate as substrate can be assayed through following the increase in absorbance at 340 nm according to the subsequent equation: