Active site
The active site of an enzyme is the region which connects the substrate and converts it into product. It is commonly a relatively small part of the overall enzyme molecule and is a 3-dimensional entity formed through amino acid residues which can lie far apart in the linear polypeptide chain. The active site is frequent a cleft or crevice on the surface of the enzyme which forms a predominantly nonpolar environment that enhances the binding of the substrate. Substrates is bound in the active site through several weak forces like van der Waals bonds, hydrogen bonds, electrostatic interactions, hydrophobic interactions and in some cases through reversible covalent bonds. Through Having bound the substrate molecule and formed enzyme–substrate complex catalytically active residues within the active site of the enzyme act on the substrate molecule to transform it first into the transition state complex and then into product, that is released into solution. Enzyme is now free to bind another molecule of substrate and starts its catalytic cycle again.
Initially two models were proposed to elaborate how an enzyme binds its substrate. In the lock, key model proposed through Emil Fischer in year 1894, the shape of the substrate and the active site of the enzyme are thought to fit together like a key into its lock in Figure. The two shapes are considered as rigid and fixed and rightly complement each other when brought together in the
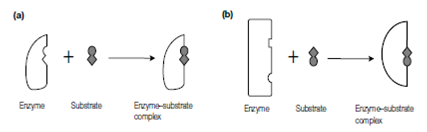
Figure: Binding of a substrate to an enzyme. (a) Lock-and-key model; (b) induced-fit model.
right alignment. In the induced-fit model proposed in the year of 1958 through Daniel E. Koshland, Jr., the binding of substrate induces a conformational change in the active site of the enzyme which is described in figure. Additionally, the enzyme may distort the substrate forcing it into a conformation similar to that of the transition state. For instance, the binding of glucose to hexokinase induces a conformational modify in the structure of the enzyme like that the active site supposes a shape which is complementary to the substrate (glucose) only after it has bound to the enzyme. The reality is which different enzymes show features of both models with some conformational and some change complementarity.