Determination of DTA peak areas:
You might have looked in Figures in which the initial and last baselines of peaks do not coincide. Thus, determination of the area under the peak might be subject to ambiguity. To resolve this problem a method for determination of the peak area is described in Figure. Both baselines are extended to a perpendicular line drawn from the maximum of the curve and the area under the two halves of the curve are determined and added to provide the total area.
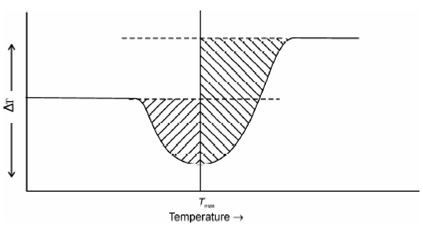
Figure: Illustration depicting the determination of DTA peak areas. The difference in the initial and final base indicates a change in heat capacity
The heat of reaction observed in DTA could be further used to calculate molar enthalpy of reactions through using the formula:
?Hm = ?Hr × Mr / m ... (11.3)
where, ?Hm = molar enthalpy of reaction, ?Hr = enthalpy of reaction, Mr = related molar mass of the compound, m = Mass of substance used for analysis.