Electrons and Protons in an Atom
An atom is the shortest particle of the basic elements which make the physical substance like solid, liquid and gas.
Each stable combination of protons and electrons make one particular kind of atom.
To understand the concepts of electronics we ought to have the understanding what is happening at the atomic level, not why this happen.
There are a lot of methods by which protons and electrons might be grouped.
They assemble in particular atomic combination for a stable arrangement.
As a consequence, the electron stays in its orbit around the nucleus.
In an atom that contains more electrons & protons than hydrogen atom, all of the protons are in nucleus, when all of the electrons are in one or more ings around the nucleus.
In the nucleus the proton makes it stable and heavier part of the atom because it is 1840 times heavier than the electron.
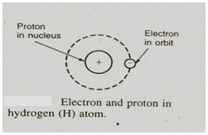
One electron is indicated as the orbital ring around the nucleus. To account for the atom's stability we might consider electron spinning around the nucleus like planets revolve around the sun.
The electrical force which attracting the electron towards proton is balanced by the mechanical force (centrifugal force) which directing it outwards.
The whole number of electrons in the outer rings ought to equal to the number of protons in the nucleus in a neutral atom.
The allocations of electrons in the orbital ring find out the atoms electrical stability. Especially significant are the number of electrons farthest from the nucleus.
For instance carbon atom illustrated in the figure, six protons in the nucleus and six electrons in two outside rings.
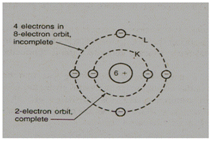
This particular outermost ring requires eight electrons stability, except, while there is just one ring which requires just 2 electrons for its stability.
As another instance, the copper atom in figure shown below contains only one electron in the last ring which might include 8 electrons.
So the outside ring of copper is less stable in comparison of carbon.
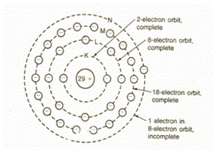
While there are several atoms closed in a copper wire, the outermost electrons are not certain from which atom they connect to.
They might migrate simply from one atom to another at random.
These electrons are known as "free electrons".