Ionic bonding:
Ionic bonding occurs among molecules having opposite charges and includes an electrostatic interaction among the two opposite charges. The functional groups that most simply ionize are amines and carboxylic acids.

Figure: (a) Ionization of an amine; (b) ionization of a carboxylic acid.
Ionic bonding is probable among a molecule consisting of an ammonium ion and a molecule consisting of a carboxylate ion. Some significant naturally occurring molecules consist of both groups - the amino acids. Both of these groups are ionized to create a structure termed as a zwitterion (a neutral molecule bearing both a positive (+ive) and a negative (-ive) charge) and intermolecular ionic bonding can occur.
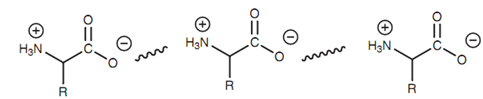
Figure: Intermolecular ionic bonding of amino acids.