Reactor coolant system:
In the event that a huge quantity of air is injected within the reactor coolant system, the inventory of dissolved hydrogen would be quickly depleted through Reaction (3-13). There could be oxygen remaining in the coolant after depletion of the hydrogen if the amount of air injected is sufficiently large. In that case, another reaction is available to the nitrogen and oxygen within the air.
radiation
2N2 + 5O2 + 2H2O ↔ 4HNO3 (3-16)
Nitric acid (HNO3) produced through this reaction will neutralize some base hold in the coolant, and if enough acid is generate, the coolant will obtain an acidic pH.
Generally, the amount of hydrogen managed in the reactor coolant, in conjunction along with other precautions employed, greatly decreases the probability in which the amount of oxygen entering the coolant will be enough to lead to Reaction (3-16). One solution would be to add more hydrogen if a huge amount of air was accidentally added to the reactor coolant. Added hydrogen would react along with remaining oxygen, disrupting the equilibrium of Reaction (3-16) causing the reverse step of in which reaction to occur. While all the oxygen has been removed, H2 and N2 could react through Reaction (3-14) and help reestablish a basic pH. The relationship among these reactions and pH subsequent the initial oxygen addition, and following hydrogen adding, is described in below figure.
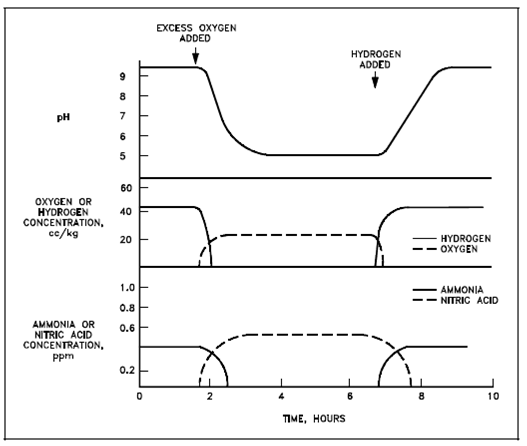
Figure: Change in pH, Gas Concentration, and Nitrogen Compounds with Excess Oxygen Added