Net result:
The net result of these reactions is easily the decomposition of water. If H2 and O2 are permitted to escape from solution as gases, a reaction continues as written. If therefore, the water is holds within a closed system under pressure (as in a reactor coolant system), H2 and O2 are confined, and an equilibrium state is reached since radiation just causes the reverse of Reaction (3-2) to take place. Primarily neutron and gamma radiation induce both the decomposition of water and the recombination of H2 and O2 to form water. Therefore, it is suitable to write Reaction as a radiation-induced equilibrium reaction.
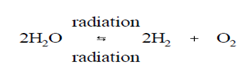
To arrive at the whole effect of radiation on water, the above procedure included the assumption which only one reaction pathway is available to every reactive species. This was completed primarily for convenience since inclusion of each possible reaction within the summation procedure becomes rather cumbersome. Even if all the reactions are taken within account, a net result is the similar as Reaction (3-12), that is reasonable since inspection of Reactions (3-3) by (3-11) displays in which the only stable products are H2 , O2 , and H2O (H2O+ and OH- join to form water, and H2O2 decomposes at high temperature). Perhaps not as obvious, more water is consumed as is generates in these reactions, and the net result is the initial decomposition of water which proceeds until equilibrium concentrations of H2 and O2 are build.
Before discussing the effects of radiation on other procedure, chemical equilibrium within the presence of ionizing radiation should be described. Equilibrium processes within aqueous solutions are discussed briefly, that states which temperature influences the equilibrium. Ionizing radiation also influences the equilibrium of those solutions.