Emission:
During the festive season or at the times of celebrations, you must have enjoyed the beautiful display of colors through fireworks in the sky. This splendid show is an output of relaxation of excited species from higher energy to the ground level releasing the excess energy in the form of light and heat. The colors we see are electromagnetic radiations in the visible range. The excitation of the species could be brought about through a number of ways, like as, absorption of electromagnetic radiation, bombardment along with electrons or other elementary particles, exposure to the high potential alternating current, arc, spark, or a flame. The radiation emitted through an excited species is characterized in terms of an emission spectrum. Like an absorption spectrum, emission spectra might be a band spectrum or a line spectrum. Figure display a schematic emission spectrum.
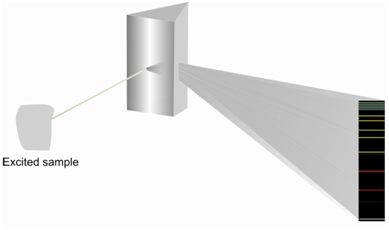
Figure: A schematic emission spectrum
The line spectrum is a result of relaxation of atoms or ions and commonly consists of a series of well described lines features of the species included. Instead, the band spectra, which consist of various groups of lines which are closely spaced and not resolved, are given through excited molecules or radicals. In case of incandescent solids we get continuous spectra whereas the line spectra and band spectra are superimposed and appear as a continuum. Now let us learn about these kinds of spectra.