Idealized representation of the three processes:
During thermal process reactions either liberate or absorb heat. Therefore, when ? H is positive (endothermic reaction), a sample heating device is energized and a positive signal is acquire; while ?H is negative the reference heating device is energized and a negative signal is acquired. An idealized representation of the three major processes observable in DSC is given in Figure. The peak area in DSC is proportional to the amount of sample and the heat of reaction and similar to DTA peak area can be expressed through following equation.
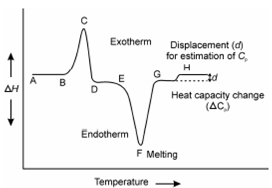
Figure: An idealized representation of the three processes observed in DSC
Peak area (A) = ± ? HmK
where ? H represents sample enthalpy change and m is the mass of sample and K is a constant called calibration factor. Dissimilar DTA it is independent of temperature. Using above Eq. 11.6, we can determine enthalpy change for a reaction directly from peak area, if we known the value of K. We can also determine enthalpy change by comparing the ? H of the sample with the known ? H of the standard. i.e.
?H s = Ak mk × ? H S/As ms
where ? Hs is the enthalpy change for sample, ? Hk is the enthalpy change for known standard, ms and mk are masses of sample and known standard respectively, and As and Ak represents the area of peaks of sample and standard materials, respectively.