Hollow cathode lamp (HCL):
It consists of a sealed cylindrical glass tube along with a quartz window at one end and a hollowed cylindrical cathode together within an anode wire made of tungsten. The cathode is fabricated from the analyte element and the lamp is filled along with an inert gas such as argon or neon under vacuum (100-200 Pa). The schematic diagram of a hollow-cathode lamp is displays in Figure. While a voltage of ~300 V corresponding to 5-50 m A current is applied among the two electrodes, a low pressure glow discharge confined to the within of the cathode material is generates.
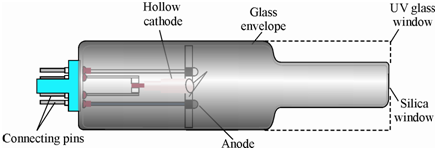
Figure: Schematic diagram of hollow cathode lamp illustrating different components
Basic function of the gas within the tube is to bombard the cathode and vapourise the atoms from the cathode surface. For that the gaseous cations are accelerated towards the cathode. Collision energy is enough to cause some atoms of the cathode to be transformed within gaseous atoms in a procedure known as sputtering. These metal atoms are then excited through collisions along with electrons and ions therefore emitting features emission lines.
The emission spectrum of the cathode material involves a number of intense, sharp lines because of transitions among excited states and the ground state, frequently known as resonance lines. Intensity of resonance lines from an HCL rises with increasing current. As of now, HCL for over 60 elements are available. Therefore, these days multielement cathode lamps are more in use for routine determinations, by their performance is not extremely reliable. In that case, cathode is made up from alloys of metals having same melting point such as Ca-Mg, Ag-Au, Cu-Fe, Zn-Cd, etc. While elements having various melting points are used, more volatile element is lost first resulting within gradual weakening of its spectrum and degeneration within one element lamp.