Radiation Sources:
All commercially available atomic absorption spectrophotometers use a radiation source in which emits the features spectrum of the element to be determined. The necessary needs of the radiation source are in which it provides a constant and intense output. Commonly two kinds of sources are in use: line sources and continuum sources. Initially continuum sources were used and the primary radiation needed was isolated along with a high resolution monochromator. Thus, these had low radiant densities and did not give sufficiently high sensitivity. Presently, the hollow- cathode lamp (HCL), belonging to the first category, has been most hugely used. The electrodeless discharge lamps (EDL) - another line sources are also often employed for the reasons and in fact are superior for the elements such as As, Se and Te along with low melting points. You have learnt about HCL and EDL in the context of atomic fluorescence spectrometry.
While the bandwidth of the primary radiation is low along with respect to the profile of the analyte absorption, a provided amount of analyte would absorb more radiation. Thus, the radiation sources having low widths of the emitted analyte lines are preferred. For that reason, a radiation sources are designed so as to operate at much lower temperatures and pressures as compared to that of the flame and furnaces used for atomisation. Since a consequence, the emitted lines are much sharper than the absorption lines to be measured. In such a set up, enough accurate measurements of the peak absorption could be made without using elaborate optics. It is referred to as source resolution. Figure describes source resolution achieved through using radiation sources emitting sharper lines.
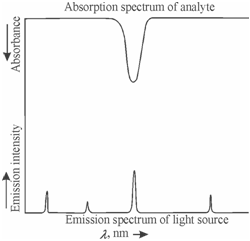
Figure: A schematic diagram illustrating the source resolution achieved by using radiation sources emitting sharper lines