The use of solvents
Even though some of the simpler types of reaction may be carried out with the reactants on their own, several reactions are taken place in solution. The most evident function of a solvent is to facilitate the mixing of solid substances, in which reaction would otherwise be very slow. Appropriate solvents rely on the nature of the compounds involved,
the most significant property being polarit. Non-polar solvents like hexane and toluene are frequently used for reactions involving organic and organoelement compounds, even though more polar coordinating solvents like ethers (including tetrahydrofuran, THF, C4H8O) are sometimes needed. For instance organomagnesium (Grignard) reagents are used and prepared in ether solution.
More polar substances usually need more polar solvents. For instance water (or sometimes ethanol) might be used for the preparation of several coordination compounds of transition metals. Water is helpful for reactions involving ionic substances, particularly when the desired product is insoluble and so may be formed directly by precipitation. An instance is
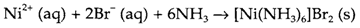
The principles considered in Topic E4 can frequently be used to choose a suitable counter-ion (for instance one of similar size) to get the desired precipitation. Solubility might also be manipulated by changing the solvent or the temperature. Hydrothermal techniques use water under conditions of high temperature and pressure to increase the solubility of reactants. Temperatures between the 150 and 500°C may be employed with pressures between 100 and 2000 bar.
Hydrothermal techniques are general for the synthesis of some solids like zeolites and also for growing single crystals of compounds like quartz, SiO2.
Though, Solvents may be themselves reactive. Sometimes solvents can be exploited, using the solvent like one of the reactants as with the following reaction in liquid ammonia:
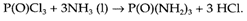
More frequently however, such type of reactivity is not desirable and solvents may require to be chosen accordingly. Both the redox and the acid/base properties of the solvents can limit the range of conditions presented and several reactions not possible in water can be carried out in other solvents. TheLiquid ammonia is good for highly basic and for reducing conditions (particularly using dissolved alkali metals). Though, for strongly acid and/or oxidizing conditions solvents like H2SO4 or liquid SO2 may be used.