Solid-state reactions
Reactions among solid substances can be very slow; since the reactants meet directly only at the interface between solid particles and the bulk reaction needs the diffusion of atoms from the solids. Even when the one reactant is liquid or gaseous the barrier to diffusion may prevent bulk reaction. For instance the formation of inert oxide films on some reactive metals like aluminum and titanium is significant for their applications. In the manufacture of the electronic devices Reactions confined to surface layers are exploited like integrated circuits made from silicon.
General ceramic synthesis of mixed oxides make use of finely divided starting materials, that ground up together and fired at high temperatures to speed up diffusion. An instance is the preparation of the 'high temperature' superconductor YBa2Cu3O6.8:
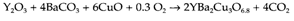
Reaction occurs at 930°C followed by cooling in O2 to give the required oxygen content of the product. BaCO3 is employed rather than BaO as this oxide is extremely sensitive to water and CO2 and so is difficult to obtain in pure form. The Ceramic synthesis is making easy by the intimate mixing of the starting materials and this can sometimes be obtained through the coprecipitation from solution of a suitable mixture of precursors. For instance a mixed oxide like CaMnO3 can be made by starting with stoichiometric quantities of calcium and manganese nitrates in aqueous solution and adding the NaOH to coprecipitate the metals like hydroxides, that is followed by firing at 1000°C.
A significant way of conquer the diffusion barrier in solid state synthesis is the method of vapor transport, in which an agent is added to the reactants to produce a volatile intermediate in a sealed tube. For instance the formation of Al2S3 is slow even at 800°C where Al is liquid and S gaseous, due to the formation of an impermeable skin of sulfide
on the surface of the metal. In the tube, by adding a trace of I2 and using a temperature gradient accelerates the reaction due to the reversible formation of volatile AlI3. The reactants are located at the hot end of the tube and the volatile iodide passes to the cooler end where the equilibrium
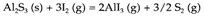
shifts back to the left and the result is formed. Specialized low-temperature methods known collectively as chimie douce ('gentle chemistry') techniques can be employed for specific types of compound.