Structure and bonding
The comprehensive explaination of a chemical structure involves specifying the relative coordinates of the atoms exist or alternatively giving all bond lengths and bond angles. A simple instance is displayed in 2. Less comprehensive information is satisfactory for most descriptive purposes. The atom's coordination number (CN) is the number of bonded atoms, irrespective of the kind (ionicity, multiplicity, etc.) of bond involved. For extremely simple molecular compounds this is clear from the formula (example O in H2O and C in CO2 (3) both have CN=2). Though, polymeric and ionic solids have greater CN values (example 4, 6 and 8, correspondingly, for the Si in SiO2, Ti in TiO2 and U in UO2), and it should not usually be assumed that the CN is given directly by the stoichiometry.

The geometrical arrangement around an atom is sometimes explained as its coordination sphere. Distinct geometrical arrangements may be explained by simple informal terms (example H2O (2) is bent and CO2 (3) linear), or by the names of polyhedra, like tetrahedra and octahedra. According to symmetry, classification is also helpful, as explained in Topic C3.
Explaining bonding in a consistent way is much harder. The word valency, meaning the number of bonds created by an atom, is helpful in simple molecular substances. Stoichiometries like CH4, CO2 and H2O can be rationalized by assuming the valencies C(4), H(1) and O(2). One can expand the idea by recognizing the possibility of variable valency; for instance, three for phosphorus in PCl3 and P2O3 and five within the PCl5 and P2O5. Unfortunately, the simple valence idea has severe limitations and can be misleading outside a narrow area. For an instance:
- it is specified that the 'normal' valencies of C and O, how can the one account for the stability of CO and the fact that it apparently has a triple bond?
- PCl5 in its solid form consists of [PCl4]+ and [PCl6]- ions. What is the valency of P here?
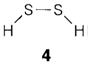
Much more severe difficulty comes into existence with transition metal compounds. For these and another reason the word valency has been largely abandoned by inorganic chemists. When it is essential to differentiate distinct stoichiometries like PCl3 and PCl5 the oxidation state is more often used. This is described according to clearer rules than valency, but as they rely on the electronegativity variation of atoms, the oxidation state can be extremely uninformative about bonding. For instance, every sulfur atom forms two covalent bonds in the compounds H2S, H2S2 (4), S2Cl2 and SCl2, and however the oxidation state of sulfur is correspondingly -2, -1, +1 and +2.
As
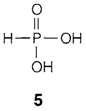
As a final instance, refer the phosphorous acid H3PO3 (5). The phosphorus' oxidation state is +3, its coordination number 4 and its valency 5. All these numbers provide helpful information but they must not be confused.