Formulae
It is significant to differentiate the stoichiometric or empirical formula of a molecular substance from its molecular formula. The previous expresses only the relative numbers of atoms exist, in the simplest possible ratio.
For instance, the compound of stoichiometry P2O5 consists of P4O10 molecules. Molecular formulae should be employed when they are known. Techniques for determining empirical and molecular formulae are described in. Alternatively, in a solid where clear molecular or other units do not present the empirical formula is usually used. For instance,
NaCl is the ionic substance and the formula does not imply that molecules are exists.
When solids consist of identifiable groups such as molecules or complex ions the formula is written to indicate this:
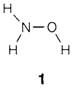
for instance, NH4NO3 is much more informative for ammonium nitrate than the empirical formula N2H4O3. This is frequently used in molecular formulae, for instance, in NH2OH (1) and Ni(CO)4, that are intended to depict the groupings of atoms exist. For coordination compounds created by transition metals formulae are written with square brackets like in [Ni(NH3)6]Br2, that indicates that six NH3 are attached directly to Ni but not the two Br. Complex ions formed by the main-group elements can be written in a identical way, for instance, [PCl4]+ and [BF4]-, even though usage is not very systematic.
While a metallic and a nonmetallic element are exist, the metallic one is all the time written first, like in NaCl and PbO2.
For the compounds among two or more nonmetals they are listed usually in the following order, based approximately on a sequence of increasing electronegativity:
For instance, we have OF2 and ClO2, so which are called oxygen difluoride and chlorine dioxide, correspondingly.
When the physical state of a substance is significant it is specified as in NaCl(s), H2O(l) and HCl(g) for solids, liquids and gases, correspondingly. (l) is assumed to be a pure liquid or the main component (solvent) in a solution. For the substances melts in water the designation (aq) (for 'aqueous') is employed. So solid sodium chloride dissolving in water is expressed:
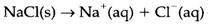
Meaning of NaCl(aq) dissolved NaCl molecules and is wrong for this substance.