Trends in Atomic Properties
The properties of the elements show the tendencies. These tendencies can be predicted using the periodic table and can be described and understood through analyzing the electron configurations of the elements. Elements contribute to lose or gain valence electrons to get stable octet formation. Stable octets are observed in the noble gases or inert gases, of Group VIII of the periodic table. Further, to this activity, there are two other significant trends. First, that the electrons are added one at a time moving from left to right across a period. As this occurs, the outermost shell's electrons experience increasingly strong nuclear attraction, therefore the electrons become closer to the nucleus and bound more tightly to it. Second, in the periodic table, moving down a column, the outermost electrons become less tightly bound to the nucleus. This occurs due to the number of filled principal energy levels (that shield the outermost electrons from attraction to the nucleus) raises downward in each group. These trends describe the periodicity that is observed in the elemental properties of electron affinity, atomic radius, electronegativity and ionization energy.
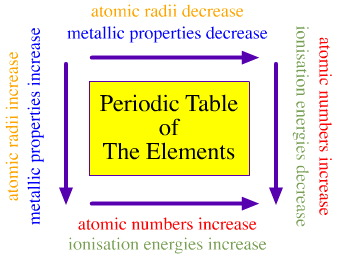
Atomic Radius
An element's atomic radius is half of the distance among the centers of two atoms of that element that are just touching each other. Usually, the atomic radius decreases across a period to the right from the left and increases down a given group. The atoms that have largest atomic radii are located in Group I and at the bottom of groups.
Across a period moving from left to right, electrons are added at a time one to the outer energy shell. Electrons in a shell cannot shield each other from the attraction to protons. Because the number of protons is also increasing, the effective nuclear charge raises across a period. This effects the atomic radius to decrease.
In the periodic table, moving down a group, numerous electrons and filled electron shells increases but the number of valence electrons left similar. The outermost electrons in a group are exposed to identical effective nuclear charge but electrons are found beyond from the nucleus like the number of filled energy shells increases. So, the atomic radii increase.