SULPHUR, SELENIUM AND TELLURIUM
The elements
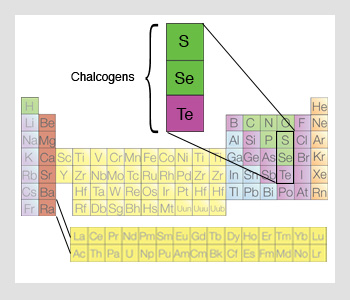
The elements known jointly as the chalcogens are in similar group (16) as oxygen. They create some compounds identical to those of oxygen, but depict many variations feature of other nonmetal groups.
Sulfur is common in the Earth's crust, taking place as metal sulfides, native, and sulfates or elemental sulfur formed through bacterial oxidation of sulfides. Several less electropositive metals known as chalcophiles are found generally as sulfide minerals; some significant instances are pyrites (FeS2), molybdenite (MoS2), cinnabar (HgS), sphalerite (zinc blende, ZnS) and galena (PbS). Volatile sulfur compounds like H2S and organic compounds are also found in petroleum and natural gas. The element is employed in large amounts used for the manufacture of sulfuric acid.
tellurium and Selenium are much rarer, found like minor components of sulfide minerals. Sulfur has various allotropic forms, the most stable of that are molecular solids consisting of S8 rings. The elemental forms of Se and Te comprise spiral chains and are semiconductors. Each atom creates two single bonds to neighbors, in all of these solids. Sulfur combines straight with oxygen and halogens (apart from I), and with several less electronegative elements to form sulfides. The other elements depict identical properties even though reactivity declines down the group.