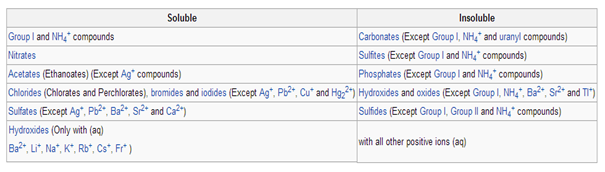
SOLUBILITY OF IONIC SUBSTANCES
Solubility is the property of a gaseous, solid, or liquid chemical substance called solute to dissolve in a solid, liquid, or gaseous solvent to create a homogeneous solution of the solute within the solvent. The solubility of a substance basically depends on the used solvent with on pressure and temperature. The scope of the solubility of a substance in a particular solvent is calculated as the saturation concentration, in which adding more solute does not raise the concentration of the solution.
Various ionic compounds (salts) dissolve in water, which arises due tothe attraction between positive and negative charges. For instance, the salt's positive ions (example Ag+) attract the partially negative oxygens in H2O. Similarly, the salt's negative ions (example Cl-) attract the partially positive hydrogens in H2O. Note: oxygen is partly negative since it is more electronegative than hydrogen, and vice-versa.
AgCl(s) 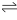 Ag+(aq) + Cl-(aq)
Ag+(aq) + Cl-(aq)
Though, there is a limit to how much salt can be dissolved in a particular volume of water. This amount is given through the solubility product, Ksp. This value relies on the type of salt (AgCl vs. NaCl, for instance), temperature, and the general ion effect.
One can compute the amount of AgCl that will dissolve in 1 liter of water, some algebra is needed.
Ksp = [Ag+] × [Cl-] (definition of solubility product)
Ksp = 1.8 × 10-10 (from a table of solubility products)
[Ag+] = [Cl-], in the nonexistence of other silver or chloride salts,
[Ag+]2 = 1.8 × 10-10
[Ag+] = 1.34 × 10-5
The result: 1 liter of water can dissolve 1.34 × 10-5 moles of AgCl(s) at room temperature. Compared with another types of salts, AgCl is poorly soluble in water. In difference, table salt (NaCl) has a higher Ksp and is, so, more soluble.