RINGS AND CLUSTERS
Introduction
Ring and cluster compounds arise in several areas of chemistry. Rings are most frequently formed by nonmetallic elements with directional covalent bonding. They include homoelement rings like S8 (1) and benzene C6H6 and ones with heteroelement bonding like S2N2 (2), borazine B3N3H6 and the silicate ion [Si3O9]3-. Clusters are polyhedral arrangements of atoms found very broadly in the periodic table: nonmetals (example P4 3 and boranes discussed below), main-group metals (example [Pb5]2- 4) and transition metals (frequently with ligands like CO; see Topic H9). Heteroelement bonding is also possible like in the A4B4 structure 5, adopted by the As4S4 (where A=As and B=S) and S4N4 (where A=S and B=N).
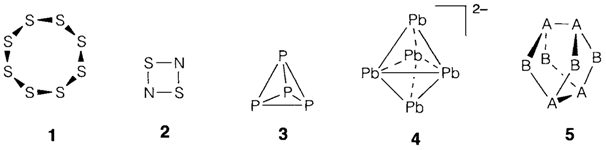
Complex molecular structures do not essentially need complex bonding models, and certainly much of organic chemistry can be understood using rather elementary ideas. Some inorganic ring and the cluster compounds like P4 and S8 can be understood in terms of elementary electron pair bonds; the octets are accomplished in each case with two nonbonding electron pairs for each S atom and one for each P. Likewise in As4S4, each such as forms three bonds and one lone-pair, each one S atom two bonds and two lone-pairs. The oppposite arrangement of S4N4 is little much harder to understand but may still be accommodated in simple ideas by placing a formal negative charge on each two-bonded N and a formal positive charge on each three-bonded S; as supposed for bonds among formal S+ entities, the S-S bonds (A-A in 5) are not normally long and weak.
Several rings and clusters, though, cannot be understood within the two-center two-electron bond framework. This is sometimes expanded by assuming resonance, for instance between the two Kekulé-type structures for benzene (6). A more natural technique is to extend the molecular orbital (MO) technique to many atoms. The so-called Hückel theory of the ring systems makes significant predictions relevant to inorganic molecules like S2N2. Clusters like boranes also require a delocalized MO approach. Wade's rules provide a helpful systematization of the principles involved and can be expanded to other systems.