PHOSPHORUS, ARSENIC AND ANTIMONY
The elements
The heavier elements in similar group (15) as nitrogen are sometimes known as 'pnictogens' and their compounds with metals like 'pnictides'. Even though the elements form some compounds identical to those of nitrogen, there are extremely pronounced variations, as is found in other nonmetal groups. Phosphorus is fairly abundant in the Earth's crust like the phosphate ion; the main mineral source is apatite Ca5 (PO4)3(F,Cl,OH), the notation (F,Cl,OH) being used to depict that F-, Cl- and OH- can be exist in varying proportions. Arsenic and antimony are much uncommon They take place in minerals like realgar As4S4 and stibnite Sb2S3, but are mainly obtained like by results from the processing of sulfide ores of other elements. Elemental P is obtained through reduction of calcium phosphate. The complex reaction estimated to:
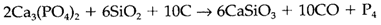
Most phosphates are employed more directly with no conversion to the element.
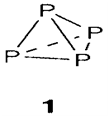
Phosphorus has several allotropes. It is most generally encountered like white phosphorus, which consists of tetrahedral P4 molecules with Td symmetry (1). Another forms, that are more stable thermodynamically but kinetically harder to make, consist of polymeric networks with three-coordinate P. The White phosphorus is highly toxic and reactive. It will combine straightly with most elements, glows in air at room temperature like a result of slow oxidation, and combusts impulsively at a temperature above 35°C. Arsenic can also form As4 molecules, but the general solid creates of this element and Sb are polymeric with three-coordination. They are noticeably less reactive than phosphorus.
Huge quantities of phosphates are employed, in detergents, fertilizers, food products, and other household products. For fertilizer applications apatite is converted through the action of acid to the very much more soluble compound Ca(H2PO4)2, termed as 'superphosphate'.