The Periodic Table
A periodic table is a tabular demonstration of the chemical elements, organized according to their atomic numbers, recurring chemical properties and electron configurations. Elements are existed in order of increasing atomic number (number of protons). The general form of table comprises an 18 × 7 grid or main body of elements positioned above the smaller double row of elements. The table can also be decomposed into four rectangular blocks: the s-block to the left, p-block to the right, d-block in the middle, and the f-block below that. The columns of the s-, d-, and p-blocks are called groups, the rows of the table are known as periods; with some of these having trivial names like the halogens or the noble gases. Because, by definition, a periodic table incorporates frequent trends, any such type of table can be employed to derive relationships between the properties of the elements and predict the properties of new, however to be discovered or synthesized, elements. The result , a periodic table-whether in the standard form or some other variant-provides a useful framework for analyzing chemical behaviour, and such types of tables are broadly use in chemistry and other sciences.
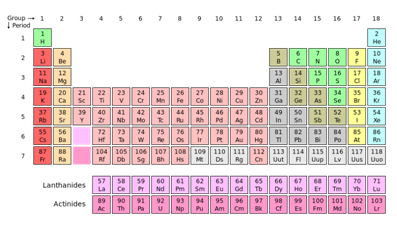
Even though precursors exist, Dmitri Mendeleev is usually credited with the publication, in year 1869, of the first extensively recognized periodic table. Dmitri Mendeleev developed his table to demonstrate periodic trends in the properties of the then-known elements. Mendeleev also supposed some properties of then-unknown elements that would be supposed to fill gaps in this table. Most of his predictions were proved right when the elements in question were consequently discovered. Periodic table of Mendeleev has since been extended and refined with the synthesis or discovery of further new elements and the development of new theoretical models to describe chemical behaviour.