OXYGEN
The element
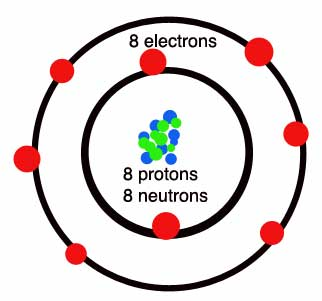
After fluorine oxygen is the second most electronegative element and creates thermodynamically stable compounds with almost all elements. It rivals fluorine in the capability to stabilize the highest known oxidation states of several elements, instances in which there is no subsequent fluoride being CIVIIO-4 and OsVIIIO4. Oxidation reactions with O2 are usually slow due to the strength of the O=O double bond (490 kJ mol-1). Oxygen is the very abundant element on Earth, making around 46% of the Earth's crust through mass. The most common minerals are complex oxides like silicates and carbonates. Oxygen is also a constituent of the water and of almost all biological molecules. Atmospheric O2 comes about entirely from photosynthesis through green plant and is not found on another known planets. Reactions involving dioxygen, both in respiration and in photosynthesis through air-breathing animals, are significant in biological chemistry. Oxygen can be taken out from the atmosphere through liquefaction and fractional distillation. At -183°C (90 K) the liquid boils and it is dangerous while mixed with combustible materials. The compressed gas is employed in metallurgy (example steelmaking) and the liquid like an oxidizer for rocket propulsion.
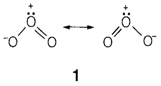
Oxygen has two allotropes, the general dioxygen O2 form and ozone O3 (1) created through subjecting O2 to an electric discharge. Ozone is a trace element of the atmosphere, in which it plays an vital role like an absorber of UV radiation.
Like predicted through molecular orbital theory dioxygen has two unpaired electrons and some of its chemistry depicts diradical characteristics; particularly, it reacts readily with another radicals. Singlet oxygen is an excited state where the two electrons in the π antibonding orbitals contain paired spins. It is formed in some chemical reactions and has dissimilar chemical reactivity.