THE NUCLEAR ATOM
The nucleus is the extremely dense region that contains neutrons and protons at the center of an atom. It was discovered in the year 1911, as a result of Ernest Rutherford's interpretation of the famous 1909 Rutherford experiment performed by Hans Ernest Marsden and Geiger, under the direction of the scientist Rutherford. The nucleus' proton-neutron model was proposed by Dmitry Ivanenko in year 1932. Approximately all of the mass of an atom is located in the nucleus, with an extremely small contribution from the orbiting electrons.
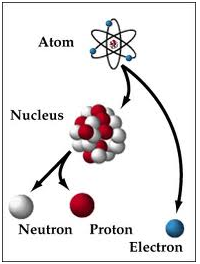
The diameter of the nucleus is within the range of 1.75 fm (femtometre) (1.75×10-15 m) for hydrogen (the diameter of a single proton) to about 15 fm for the heaviest atoms, like uranium. These dimensions are very much smaller than the diameter of the atom itself (nucleus + electron cloud), by a factor of about 23,000 (uranium) to about the 145,000 (hydrogen).
The category of physics concerned with understanding and studying the atomic nucleus that includes its composition and the forces which bind it together is called nuclear physics.