MOLECULAR SHAPES: VSEPR
Through the electron pairs covalent molecules are bonded to other atoms. The electron pairs repel the another electron pairs and attempt to move as far apart as possible in order to stabilize the molecule, Being mutually negatively charged. This repulsion causes covalent molecules to have distinct shapes, termed as the molecule's molecular geometry. There are various different techniques of determining molecular geometry. A scientific model, known as the VSEPR (valence shell electron pair repulsion) model can be employed to qualitatively guess the shapes of molecules. In this model, the AXE method is employed in determining molecular geometry by counting the numbers of bonds and electrons associated to the center atom(s) of the molecule.
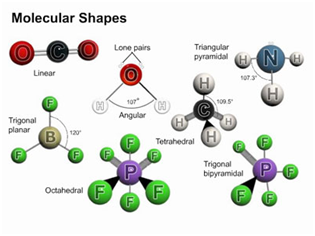
The VSEPR model is by no means is a perfect model of molecular shape! It is just a system which describes the known shapes of molecular geometry as discovered by experiment. This can permit us to predict the geometry of similar molecules, making it a fairly helpful model. Modern techniques of quantitatively calculating the most stable (lowest energy) shapes of molecules can take so many hours of supercomputer time and is the domain of computational chemistry.