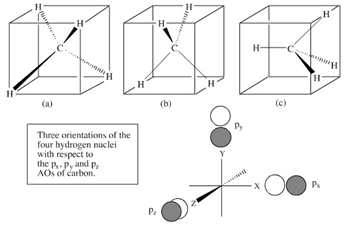
MOLECULAR ORBITALS: POLYATOMICS
A polyatomic ion, also termed as a molecular ion, is a charged species (ion) which is composed of two or more atoms, the covalently bonded or of a metal complex that can be refered as acting as a single unit in the perspective of acid and base chemistry or within the formation of salts. The prefix "poly-" means "many," in Greek, but even ions of two atoms are generally considered as polyatomic. In past literature, a polyatomic ion is also considered as a radical and less generally, like a radical group. In contemporary use, the word radical refers to free radicals that are (not necessarily charged) species with an unpaired electron.
For instance, a hydroxide ion is made of one hydrogen atom and one oxygen atom: its chemical formula is (OH)-. It has a charge of -1. An ammonium ion is made up of one nitrogen atom and four hydrogen atoms: Its chemical formula is (NH4)+. It has charge of +1.
A polyatomic ion can frequently be referred as the conjugate acid or conjugate base of a neutral molecule. For instance, the sulfate anion, SO42-, is derived from H2SO4, that can be regarded as SO3 + H2O.
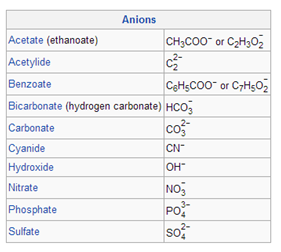
Examples of common polyatomic ions