LEWIS ACIDS AND BASES
Definition and scope
A Lewis acid is any species able of accepting a pair of electrons and a Lewis base is a species with a pair of electrons available for donation. The words acceptor and donor are also generally used. Lewis acids involve H+ and metal cations, molecules like BF3 with not complete octets, and ones like SiF4 where octet expansion is feasible. Any species with nonbonding electrons is potentially a Lewis base, including molecules like NH3 and anions like F-. The Lewis acid-base definition should not be confused with the Brønsted one: Brønsted bases are also Lewis bases and the H+ is a Lewis acid but Brønsted acids like HCl are not Lewis acids.
To give a donor-acceptor complex Lewis acids and bases may interact; for instance, The bond created is sometimes denoted through an arrow (as in 1) and called a dative bond but it is not actually distinct from any other polar covalent bond. So the complex [SiF6]2- has a regular octahedral structure where the two 'new' Si-F bonds are impossible to differentiate from another four. (It is isoelectronic with SF6)
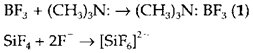
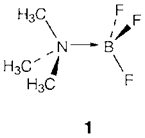
The range of the donor-acceptor concept is very broad and encompasses several types of chemical interaction, including the solvation and complexation of metal ions and the formation of coordination compounds by transition metals. Several chemical reactions also rely on donor-acceptor interactions. For instance, the hydrolysis of SiCl4 to give Si(OH)4 in water begins with a step like
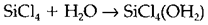
In which H2O is acting as a donor to the SiCl4 acceptor.