Lanthanum and the Lanthanides
The elements
The lanthanides are 14 elements that are following lanthanum in the periodic table, and related with the filling of the seven orbitals of the 4f shell. The symbol Ln is frequently used to represent these elements collectively. Atomic configurations are difficult with electrons in 4f, 5d and 6s orbitals which are outside the Xe core. From the whole ionization energies the first three are comparatively low, that are leading to electropositive metals along with chemistry dominated through the Ln3+ state in solution and in ionic solids. All Ln3+ ions contain electron configurations (4f)n, but the 4f orbitals are very contracted in size and not overlap considerably with neighboring atoms. Different from the case along with the d orbitals in the transition elements, magnetism and spectra related along with 4f orbitals in Ln3+ compounds are very identical to those found in free gas-phase ions. Ligand field and chemical bonding influences related with incomplete 4f orbitals are extremely small and hardly detectable in chemical trends. So the chemistry of all Ln3+ ions is very identical and distinguished only through the gradual contraction in radius related with increasing nuclear charge. The lanthanide contraction is also significant for the transition elements of the 5d series.
The oxidation states that are +2 and +4 are found for some elements, follow the trend in ionization energies across the series, that depict patterns similar to those found in configurations of p and d electrons. The third ionization energy increases from La to Eu and then a drop takes place after the half-filled shell (Eu2+, 4f7). The increment then carry on to Yb, and drops at Lu since the 4f shell is filled and the electron ionized is in 5d. Fourth ionization energies (that are substantially larger) depict a identical pattern displaced through one element, so increasing from Ce to Gd and falling to Tb.
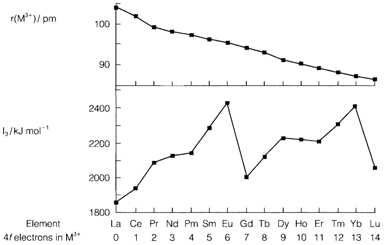
Fig. 1. Ionic radius of M3+, third ionization energy I3, and number of 4f electrons in M3+ for the elements La-Lu.
Promethium is a radioactive element along with a half-life of 2.6 years and does not take place naturally. The other elements, recognized sometimes like the rare earth elements, are all the time found in association, mainly in the minerals monazite (LnPO4) and bastneasite (LnCO3F). The electropositive and reactive elements can be got through the reduction of LnCl3 with Ca, and are occasionally used together like 'mischmetal'. Specialist applications of the individual lanthanides rely on the spectroscopic properties of Ln3+ ions (example Nd in lasers) and on the magnetic properties of some of the elements (example Sm). The ions can be separated through ion-exchange chromatography from aqueous solution, by using the difference of complexing properties beyond the series.