Transition Metals
In chemistry, the word transition metal (sometimes also known as a transition element) has two feasible meanings:
The IUPAC definition says that a transition metal is "an element whose atom comprose an incomplete d sub-shell, or that can provide rise to cations with an incomplete d sub-shell".
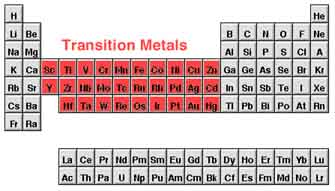
Most scientists explain a "transition metal" like any element in the d-block of the periodic table, that involves groups 3 to 12 on the periodic table. In the d-block all elements are metals. Acyually, the f-block is also involved in the form of the actinide and lanthanide series.
Jensen has reviewed the history of the word transition element (or metal) and d-block. The word transition was firstly used to explain the elements now known as the d-block via the English chemist Charles Bury in 1921, who considered a transition series of elements through the change of an inner layer of electrons (for instance n=3 in the 4th row of the periodic table) from a stable group of 8 to one of 18, or from 18 to 32.