INTRODUCTION TO NONMETALS
Non-metal or Nonmetal, is a term used in chemistry while classifying the chemical elements. On the basis of their usual chemical and physical properties, each element in the periodic table can be known either a metal or a nonmetal. (Some elements with intermediate properties are considered as metalloids).
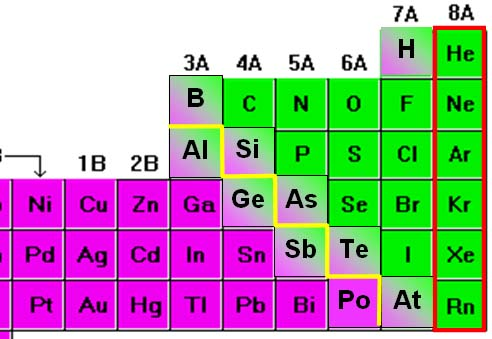
The elements usually regarded as nonmetals are:
- In Group 1: hydrogen (H) (The only nonmetal within this group)
- In Group 14: carbon (C) (Again, the only nonmetal within this group)
- In Group 15: (the pnictogens): nitrogen (N), phosphorus (P)
- In Group 16: (the chalcogens): oxygen (O), sulfur (S), selenium (Se)
- In Group 17: (the halogens): fluorine (F), chlorine (Cl), bromine (Br), iodine (I)
- All elements (with the feasible exception of ununoctium) in Group 18 - the noble gases
There is no exact definition for the term "nonmetal" - it covers a common spectrum of behaviour. General properties considered feature of a nonmetal include:
- poor conductors of electricity and heat when compared to metals
- they form acidic oxides (where metals usually form basic oxides)
- in solid form, they are brittle and dull, rather than metals that are lustrous, ductile or malleable
- generally have lower densities than metals
- they have considerably lower melting points and boiling points than metals (with the exception of carbon)
- non-metals contain high electronegativity
Only eighteen elements in the periodic table are usually referred as nonmetals, compared to over eighty metals but nonmetals make up mainly the crust, oceans and atmosphere of the earth. Bulk tissues of living organisms are composed approximately entirely of nonmetals. Several nonmetals are monatomic noble gases or create diatomic molecules in their elemental state, not like metals that (in their elemental state) do not form molecules at all.