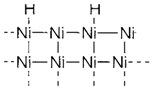Industrial Chemistry: Catalysts
Introduction
A substance that speeds up a chemical reaction but is not itself consumed is known as a catalyst. Catalysts do not change the position of equilibrium or the thermodynamics of a reaction, but act through giving a substitute pathway of lower activation energy. A high proportion of the industrial chemical processes organic and inorganic, make use of catalysts. They permit several reactions to be gets performed at lower temperatures than without a catalyst, and also give selectivity in creating a particular product in reactions in which various products are possible thermodynamically. Enzymes (that often consist of metallic elements) are exclusively selective biological catalysts.
A catalyst available in similar phase as the reactants (usually liquid) is called homogeneous, one in a dissimilar phase is heterogeneous. Several heterogeneous catalysts are solids, and act through adsorption of liquid or gaseous reactants on a surface. Homogeneous catalysts are particular molecules, frequently organometallic compounds, which can be tailored in a more particular way to provide a needed result than is possible with heterogeneous catalysts. Alternatively, it is usually harder to split the results from homogeneous catalysts. Other variation is that heterogeneous catalysis is generally performed at higher temperatures than homogeneous catalysis. Metallic elements employed like heterogeneous catalysts are frequently in the form of small particles on a support like Al2O3 or SiO2. This gives a high surface area for the active catalyst, and reduces the trend for sintering (that is coalescence into big particles) at the operating temperature.
A catalytically active substance might be capable to bind the reactant molecules and release the products. For instance, hydrogenation along with a metallic Ni catalyst carries on through adsorbed hydrogen atoms formed through dissociation of H2 on the metal surface (1). Transition metals and its compounds are efficient catalysts due to their capability to coordinate molecules and to alter oxidation state. A suitable degree of reactivity is frequently provided through elements from groups 9 and 10, either within metallic form or as organometallic compounds. Much more reactive elements (example early transition metals) are very much similar to bind reactants irreversibly, less reactive ones (example Au) not to bind them at all.