Industrial chemistry: Bulk Inorganic Chemicals
Production
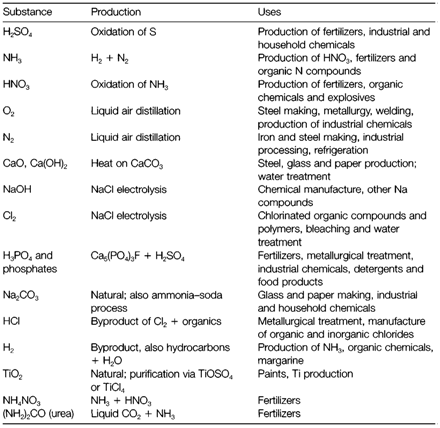
Table 1 depicts a selection of the main inorganic chemicals which are produced in annual quantities of several millions of tonnes. Substances that are made in comparable amounts which are not listed include fuels and organic chemicals created from petroleum, and construction metals like iron. In several cases the basic chemical reactions employed to generate the compounds in Table 1 are simple, even though catalysts are frequently needed. The design of processes to create the most economical use of raw materials and energy, and to minimize polluting wastes, is, though, not simple. The raw materials required involve air (for N2 and O2), sulfur (mined like native S or acquires from processing sulfide minerals), natural gas and oil (is a source of energy and H2), NaCl, and the calcium carbonate and phosphate. It is interesting to refer some facts of the chlor-alkali industry, one of the very old sectors of the chemical industry that links the manufacture of Cl2, NaOH and Na2CO3. The source material, NaCl, is employed in greater amounts than any other raw material in the complete inorganic chemical industry.
Cl2 is produced through electrolysis of NaCl. A molten NaCl-CaCl2 mixture provides metallic Na and Ca at another electrode; these metals are employed industrially; for instance, like reducing agents for production of other electropositive metals like Ti. Very much greater quantities of NaCl are electrolyzed within aqueous solution to provide NaOH, with H2 like a byproduct. Cells along with a mercury electrode provide Na-Hg amalgam like the initial product that is then reacted with water; modern diaphragm cells providing aqueous NaOH directly are cheaper to run and prevent the use of toxic mercury.
For economic basis the amount of NaOH produced relies on the demand for Cl2. Though, NaOH and Na2CO3 are interchangeable in several uses (example. glass and paper manufacture), and so any short-fall in NaOH production could be made up through the carbonate. A few Na2CO3 is obtained from natural deposits but it is also prepared synthetically through the ammonia-soda or Solvay process. The entire reaction,
2NACL + CaCO3 → CaCI2 + Na2CO3
is thermodynamically not favourable but can be achieved in various steps:
1. NaCI + NH3 + H2O + CO2 → NH+4 (aq) + CI- (aq) + NaHCO3(s)
2. 2NaHCO3(s)→Na2CO3(S)+ CO2(g)+H2O(g)
3. CaCO3(s)→CaO(s) + CO2(g)
4. CaO(s)+2NH4+ (aq) + 2CI-(aq)→ Ca2+(aq) +2CI-(aq) + 2NH3(g)+H2O
NH3 and CO2 are recycled. The equilibrium is shifted in favor of the results through providing heat in the endothermic stages (ii) and (iii). The complete energy that is needed comes from burning coke, mixed with CaCO3 in stage (iii), and a few heat is also recycled from the exothermic steps (i) and (iv).