HYDROGEN
The element
Hydrogen is the most common element in the Universe and is a main constituent of stars. It is comparatively much less general on Earth but nevertheless creates nearly 1% through mass of the crust and oceans, mainly like water and in hydrates and hydroxide minerals of the crust. It is everywhere in biology.
Under general conditions the dihydrogen molecule H2 is the stable form of the element, even though atomic hydrogen can be made within the gas phase at high temperatures, and hydrogen may turn into a metallic solid or liquid at very high pressures. At the 1 bar pressure, dihydrogen condenses to a solidifies at 14 K and liquid at 20 K, these being
the lowest melting and boiling points for any substance apart from helium. The H-H bond has a length of 74 pm and a dissociation enthalpy of 436 kJ mol-1. This is the most short bond known and one of the strongest single covalent bonds. Even though it is thermodynamically able of reacting with many compounds and elements, these reactions frequently have a large kinetic barrier and need elevated temperatures and/or the make use of catalysts.
Dihydrogen is an significant industrial chemical, mainly made from the steam re-forming of hydrocarbons from natural and petroleum gas. The most simple of these reactions,
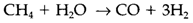
is endothermic and temperatures almost 1400 K are required to shift the equilibrium to the right. Most important uses of hydrogen are in the synthesis of ammonia, the hydrogenation of vegetable fats to create margarine, and the production of Hydrogen chloride and organic chemicals.