GROUP 13: ALUMINUM TO THALLIUM
The elements
The elements indium, aluminum, gallium and thallium comprise valence electron configurations (ns)2(np)1 and their chemistry is dominated through the +3 oxidation state for the lighter elements. Though, the group trends are extremely different, from those in groups 1 and 2. The Al3+ ion has a large charge/radius ratio and is polarizing strongly, so that important deviations from simple ionic behaviour are frequently observed. The filling of the d shells (and 4f in period 6) leads to decreased electropositive character for Ga, In and Tl identical to that displayed in group 12. There is also an advance stabilization of lower oxidation states down the group.
Aluminum is the most common metallic element in the Earth's crust, being a constituent of approximately all silicate minerals. Weathering leaves deposits of the extremely insoluble aluminum minerals Al(OH)3 and AlO(OH), known together as bauxite, that creates the main source of the element. The metal is extracted through electrolysis of fused cryolite Na3[AlF6]. Even though reactive when clean, the metal simply creates a very resistant oxide film, that permits widespread applications as a lightweight construction material and in cooking and other vessels.
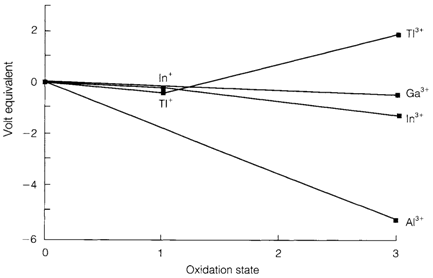
Fig. 1. Frost diagram showing the oxidation states of Al, Ga, In, Tl in aqueous solution at pH=0.
Ga, In and Tl are less common elements, acquired in small amounts from sulfide minerals of other elements and employed only in particular applications. The metals are less reactive than aluminum; Fig. 1 depicts a Frost diagram where the much larger negative slope (negative electrode potential;) of Al is apparent. Thallium compounds are very toxic but do not generally pose an environmental hazard since they are little used.