Complexes: π Acceptor Ligands
Definition and evidence
Several ligands have a nonbonding electron pair which can act like a donor to empty orbitals on the metal atom. In ligands termed like π acceptors or π acids a donor-acceptor interaction also occurs in the opposite direction. If a ligand having empty orbitals of π type symmetry regarding to the bond axis these might act like acceptors for electrons in filled metal orbitals of the right symmetry. This is identified as back donation. The most simple and most common π acid ligand is carbon monoxide CO. This works like a donor in the general way, via the occupied lone-pair orbital centered on carbon (the 3σ MO;). The π antibonding orbital could also interact with filled d orbitals to provide the π-acceptor interaction (Fig. 1). Sometimes the combination of σ-donor and π-acceptor interaction is explained as synergic, like the electron flows in reverse directions make possible each other. Proof for the π-acceptor interaction comes from several sources.
- CO and associated ligands stabilize extremely low oxidation states of transition elements, frequently zero. π- Acceptor interactions eliminate electron density from a metal atom and make it possible a lower oxidation state than is generally found with ligands like water and ammonia.
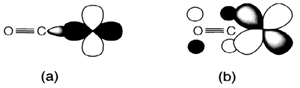
Fig. 1. Bonding in CO complexes showing (a) σ overlap of CO lone-pair with empty metal d orbital, and (b) overlap of CO π* with occupied metal d orbital.
- Of the π antibonding orbital the partial occupation in CO weakens the bond. This is very easily displayed from the bond stretching frequency measured through IR spectroscopy. CO stretching frequencies within carbonyl compounds are almost all the time less than in free CO, and also decrease in a sequence like
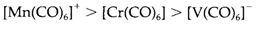
In which for back donation is increasing the availability of metal electrons. (some CO complexes, example BH3CO and Au(Cl)CO, have stretching frequencies little higher than in the free molecule, representative that little or no π interaction is occurring in these cases.)
π-acceptor properties in another ligands might be judged through their ability to stabilize low oxidation states in a identical way to CO, or through their influence on the CO stretching frequency while placed in similar complex. Two π-acceptor ligands within a trans configuration will compete for similar d orbitals. Placing a strong π acceptor trans to CO will thus lessen the accessibility of electrons for back-bonding and so the CO stretching frequency will be higher than or else. By this basis the following order of π-acceptor strength has been deduced for a few ligands:
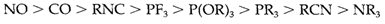
π back-bonding with phosphines is normally assumed to include valence expansion on the phosphorus. As supposed, the strength increases along with the electronegativity of the attached groups. Even though nitrogen ligands like pyridine (in which N is part of an aromatic ring system) are π acceptors, amines R3N are not, as nitrogen cannot enlarge its valence shell.