Introduction
The periodic table was invented by Mendeleev in response to observed regularities in the chemistry of the elements before there was any understanding of their electronic basis. His technique was vindicated by his capability to predict the properties and simple chemistry of the then not known elements germanium and gallium by simple interpolation among known elements in neighboring positions. So, Chemical periodicity was seen to be a powerful tool in the interpretation and even prediction of the chemical properties of elements.
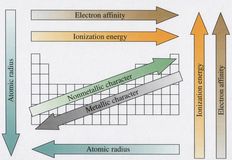
Because Mendeleev the variety of chemical compounds known has extended enormously and it has become apparent such simple interpolation methods have several limitations. In a few groups (particularly the s block) the chemistry is quite similar and mainly the observed trends in the group can be interpreted easily from changes of atomic properties like radius. Though, in the p and d blocks, this is not so simple. Complications occur partly from the reality that atomic trends are themselves less regular (due to the way in which the periodic table is filled) and partly from the greater complexities in chemical bonding, which reply in a more subtle way to changes in orbital size and energy.
The periodic table remains the most significant framework for understanding the comparative chemistry of elements and many major trends can be understood from the atomic trends explained. Though, most elements have peculiarities, which even though they can be rationalized in terms of periodic trends, would possibly not have been predicted if they were not known.