Bond Strength
In chemistry, bond strength is the degree at which each atom joined to another atom in a chemical bond contributes to the valency of this another atom. Bond strength is closely related to bond order and can be quantified through:
1. bond energy: needs lengthy calculations, even for the most simple bonds.
2. bond-dissociation energy
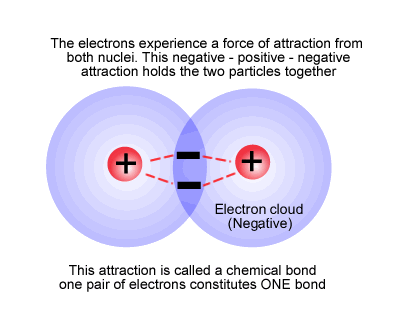
Other criterion of bond strength is the qualitative relation among bond energies and the overlap of atomic orbitals of the bonds (Mulliken and Pauling). The more these overlap, the more the bonding electrons are to be found among the nuclei and thus stronger will be the bond. Overlap is essential for the creation of molecular orbitals. This overlap can be measured and is called the overlap integral.