Atomic orbital
An atomic orbital is a mathematical function that explains the wave-like behaviour of either one electron or a pair of electrons in an atom. This function can be employed to calculate the probability of finding any electron of an atom in any particular region around the atom's nucleus. The word may also consider to the physical region where the electron can be calculated to be, as described by the specific mathematical form of the orbital.
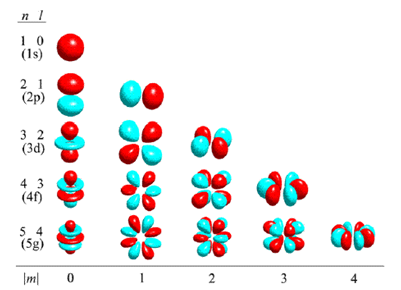
Atomic orbitals are usually categorized by n, m, and l quantum numbers, which correspond to the electron's energy, an angular momentum vector component and angular momentum, correspondingly. Each orbital is defined by a different set of quantum numbers (n, l, and m) and contains the maximum of two electrons each with their own spin quantum number. The simple names are s orbital, p orbital, d orbital and f orbital consider to the orbitals with angular momentum quantum number l = 0, 1, 2 and 3 correspondingly. These names points out the orbital shape and are employed to explain the electron configurations. They are derived from the features of their spectroscopic lines: sharp, principal, diffuse and fundamental, others being named in alphabetical order.
Atomic orbitals are the fundamental building blocks of the atomic orbital model (on the known as the electron cloud or wave mechanics model), on the other hand, a modern framework for visualizing the electrons' microscopic behaviour in matter. The electron cloud of a multi-electron atom may be seen as being built up (in approximation) in an electron configuration that is a result of simpler hydrogen-like atomic orbitals, in this model. The repeating periodicity of blocks of the 2, 6, 10, and 14 elements within sections of the periodic table arises naturally from the total number of electrons which occupy a entire set of s, p, d and f atomic orbitals, correspondingly.