GROUP 1: ALKALI METALS
The elements
The elements of group 1 are together known as alkali metals after their hydroxides' alkaline properties like NaOH. The atoms comprise the (ns)1 electron configuration and the M+ ions so they are easily formed. Alkali metals are the mainly electropositive of all elements, and their compounds between the most ionic. Some group trends are displayed in Table 1. Approximately constant electropositive character is preserved down the group through parallel fall in ionization, atomization and lattice or hydration energies. Lithium is little bit differs from the rest of the series, in some respects. The solubilities and the thermal stabilities of its compounds follow patterns which are more identical to those of group 2 elements than to those of the rest of group 1. This diagonal relationship can be recognized from the small size of the Li+ cation that leads to trends in lattice energies and solvation energies more similar to those of the higher charged ions in group 2.
Just sodium and potassium are fairly abundant on Earth and are main elements of life. They take place in several silicates, but weathering reactions at the Earth's surface guide to the dissolution of the extremely soluble cations that are general in sea water and are eventually deposited in halide minerals like NaCl and KCl. Cs, Li and Rb are of lower abundance, and acquired from silicate minerals. Francium is radioactive. It is longest-lived isotope 223Fr has a half-life of only 22 min and takes place in remarkably small amounts in uranium minerals.
Table 1. Properties of alkali metals: melting and boiling points, atomization and ionization enthalpies, ionic radii and standard electrode potentials
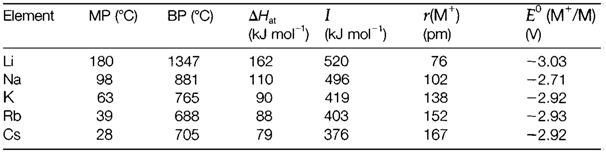
The elements are soft low-melting metals and extremely strong reducing agents, reacting violently with several substances. Their main applications are like compounds (particularly carbonate, hydroxide and sodium chloride) but the elements can be prepared through electrolysis of sodium metal and fused halides is employed in industrial processes like the production of metallic Ti.