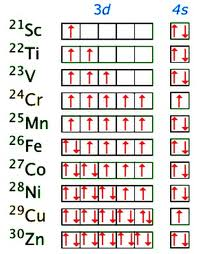
3d Series: Aqueous Ions
In aqueous solution a metal ion is a cation, dissolved in water, of chemical formula [M(H2O)n]z+. The solvation number, n, determined through a range of experimental techniques is 4 for Li+ and Be2+ and 6 for elements in rows 3 and 4 of the periodic table. Actinide and Lanthanide aqua ions comprise solvation number of 8 and 9. The strength of the bonds among the metal ion and water molecules in the main solvation shell get increases along with the electrical charge, z, on the metal ion and reduce due to its radius, r, get increases. Aqua ions are matter to hydrolysis.
The first hydrolysis constant's logarithm is proportional to z2/r for mainly aqua ions. The aqua ion is related, during hydrogen bonding with other water molecules in a secondary solvation shell. In the first hydration shell Water molecules substitute along with molecules in the second solvation shell and molecules in the bulk liquid. In the first shell the residence time of a molecule changes among the chemical elements from about 100 ps to more than 200 y. Aqua ions are famous in electrochemistry.