Compute the number of normal modes of vibration:
Problem:
Compute the number of normal modes of vibration for benzene.
Draw the vibrational modes of CO2 molecule using their given figureand comment on their IR activity.
Answer:
Since benzene is not a linear molecule and has twelve atoms in it, the number of vibrational modes will be [ (3 × 12) - 5 = 31].
The vibration modes of carbon dioxide can be drawn as follows:
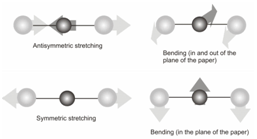
Out of these all the modes other than the symmetric stretching mode will be IR active.