Example:
0.32 g stainless steel sample was dissolved in nitric acid and the resulting solution was made to 100 cm3 with water. There are five standards and the sample solution was aspirated within flame for the determination of nickel. The subsequent observations were made.
Concentration of Nickel (ppm) Absorbance
2 0.126
4 0.250
6 0.374
8 0.500
10 0.626
Sample 0.226
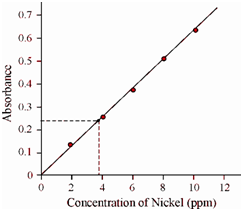
Calculate the percentage of nickel in steel sample.
Solution
Let us prepare the calibration plot by taking the concentration on X-axis and absorbance on Y-axis, as display as follows According to the calibration plot the sample concentration is found to be 3.612 ppm. That is corresponds to 1.12%.