Solvation effects:
Once the alkyl ammonium ion is created, it is solvated via water molecules - a process that involves hydrogen bonding among the oxygen atom of water and any N-H group available in the alkyl ammonium ion. Water solvation is a stabilizing factor that is as significant as the inductive effect of alkyl substituents and the more hydrogen bonds that are possible, the greater the stabilization.
Solvation is much stronger for the alkyl ammonium ion formed from a primary amine as compared for the alkyl ammonium ion created from a tertiary amine. This is since the former ion has three N-H hydrogens obtainable for H-bonding, compared with only one such type of N-H hydrogen for the latter. The result of it is that there is more solvent stabilization experienced for the alkyl ammonium ion of a primary amine as compared to that experienced through the alkyl ammonium ion of a tertiary amine. The meaning of this is that tertiary amines are usually weaker bases than primary or secondary amines.
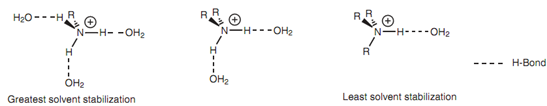
Figure: Solvent effect on alkyl ammonium ions from primary, secondary, and tertiary amines.