Point imperfections:
Point imperfections in crystals could be separated into three major defect categories. They are described in below Figure.
1. Vacancy defects result from a missing atom in a lattice position. A vacancy type of defect could result from imperfect packing in during the crystallization procedure, or it might be because of increased thermal vibrations of the atoms brought about through elevated temperature.
2. Substitutional defects result from an impurity present at a lattice position.
3. Interstitial defects result from an impurity located at an interstitial site or one of the lattice atoms being within an interstitial position alternatively of being at its lattice position. Interstitial refers to locations among atoms in a lattice structure.
Interstitial impurities called network modifiers act as point defects in amorphous solids. A presence of point defects could enhance or lessen the value of a material for engineering construction depending upon the intended use.
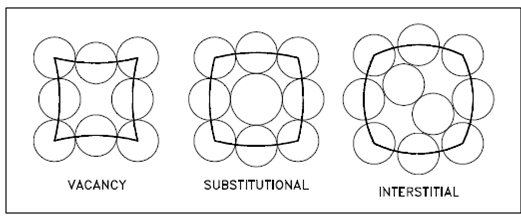
Figure: Point Defects