More substituted carbocation:
The meaning of this interaction is that the 2p orbital is not completely vacant because the σ electrons of the C-H bond can spend a small amount of time entering the space taken by the 2p orbital. The meaning of this is that the C-H bond becomes slightly electron deficient. The result of it is the positive charge is delocalized and therefore stabilized. The much more alkyl groups attached to the carbocation, the more possibilities there are for hyperconjugation and much more stable the carbocation. For instance, the more substituted carbocation can be stabilized through the hyper conjugation to nine C-H bonds, while the less substituted carbocation can just only be stabilized through hyperconjugation to one C-H bond.
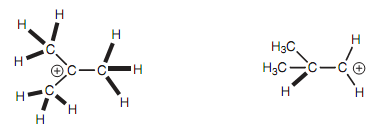
Figure: (a) More substituted carbocation; (b) less substituted carbocation.