Hydrides of nonmetals
Hydrogen creates molecular compounds with nonmetallic elements. Table 1 depicts a selection. With the exclusion of the boranes hydrogen all the time forms a single covalent bond. Complexities of formula or structure occur by the possibility of catenation, direct element-element bonds like in hydrogen peroxide, H-O-O-H, and in several organic compounds. The IUPAC (International Union of Pure and Applied Chemistry) has suggested systematic names ending in -ane, but for several hydrides 'trivial' names are still usually used. Additionally to binary compounds, there are several others with many elements present. These include almost all organic compounds, and inorganic examples like hydroxylamine, H2NOH. The naming inorganic compounds' substitutive system derived from hydrides is identical to the nomenclature used in organic chemistry (example chlorosilane, SiH3Cl;). Table 1 depicts the bond strengths and the standard free energies of formation of hydrides. Bond strengths and thermodynamic stabilities reduce down each group. Compounds like silanes and boranes are strong decreasing agents and may inflame impulsively in air. Reactivity usually increases with catenation.
Table 1. A selection of nonmetal hydrides (E indicates nonmetal)
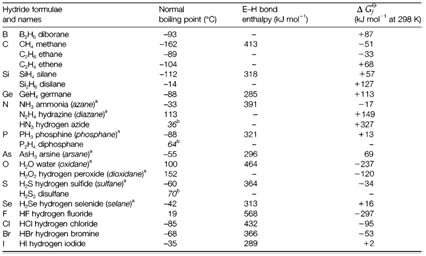
aIUPAC recommended systematic names which are seldom used.
bExtrapolated values for compounds decomposing before boiling at atmospheric pressure.
Usual routes to the preparation of hydrides include:
(i) direct combination of elements:
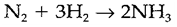
(ii) reaction of a metal compound of the element with a protonic acid like water:
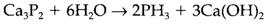
(iii) reduction of a oxide or halide with LiAlH4 or NaBH4:
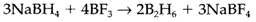
Route (ii) or (iii) is needed when direct combination is thermodynamically not favourable. Catenated hydrides can frequently be created through controlled pyrolysis of the mononuclear compound. Brønsted acidity occurs from the possibility of transferring a proton to a base, that may sometimes be the same compound. Basicity is feasible when nonbonding electron pairs are exist. Basicity in the direction of protons decreases in the direction of the right and down each group in the periodic table, that's why ammonia is the strongest base between simple hydrides.