Hydrides of metals
All metallic elements not form hydrides. Those that form hydrides may be classified as follows.
Highly electropositive metals have solid hydrides frequently considered as containing the H- ion. They have structures identical to halides, even though the ionic character of hydrides is certainly much lower. Instances include LiH (rocksalt structure) and MgH2 (rutile structure;). Some d- and f-block elements form hydrides which are usually metallic in nature, and of variable (nonstoichiometric) composition. Instances include TiH2 and CeH2+x.
A number of heavier p-block metals form molecular hydrides identical to those of nonmetals in similar group, instances being digallane (Ga2H6) and stannane (SnH4), both of extremely low stability.
Hydrides of more electropositive elements that can be made by direct reaction among elements. They are extremely strong reducing agents and react with water to provide dihydrogen:
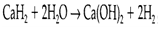
The hydride ion can proceed like a ligand and form hydride complexes identical in some ways to those of halides, even though their stability is frequently limited by the reducing properties of the H- ion. The most significant complexes are the tetrahedral ions BH4-and AIH4-generally found like as the salts NaBH4 and LiAlH4. They might be made by the action of NaH or LiH on a halide or identical compound of B or Al, and are employed as reducing agents and for the preparation of hydrides of other elements.
Several transition metal complexes consisting of hydrogen are identified, including the unusual nine-coordinate ion [ReH9]2 - (see Topic H5). Hydride is a extremely strong σ-donor ligand and is frequently found in conjunction with π-acid ligands and in organometallic compounds.