Sources of hydrogen:
Sources of hydrogen causing embrittlement have been encountered in the making of steel, in processing parts, within welding, within storage or containment of hydrogen gas, and associated to hydrogen as a contaminant in the environment that is frequently a by-product of general corrosion. It is the latter which concerns the nuclear organization. Hydrogen might be generates through corrosion reactions like as rusting, electroplating, and cathodic protection. Hydrogen might also be added to reactor coolant to erased oxygen from reactor coolant systems.
As display in Figure, hydrogen diffuses along the grain boundaries and merges along with the carbon (C) that is alloyed along with the iron, to form methane gas. The methane gas is not mobile and collects in small voids along the grain boundaries where it builds up enormous pressures which initiate cracks. A Hydrogen embrittlement is a primary reason in which the reactor coolant is managed at a neutral or basic pH in plants without aluminum elements.
If the metal is under a high tensile stress, brittle failure could occur. At general room temperatures, the hydrogen atoms are absorbed within the metal lattice and diffused by the grains, tending to gather at inclusions or other lattice defects. The path is transgranular if stress induces cracking under these conditions. At high temperatures, the absorbed hydrogen tends to gather in the grain boundaries and stress-induced cracking is then intergranular. A cracking of martensitic and precipitation hardened steel alloys is believed to be a form of hydrogen stress corrosion cracking that results from the entry into the metal of a portion of the atomic hydrogen which is generates in the subsequent corrosion reaction.
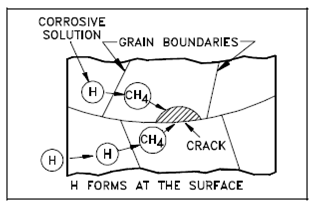
Figure: Hydrogen Embrittlement
3 Fe + 4 H2O→ Fe3O4 + 4 H2
Hydrogen embrittlement is not a permanent condition. The hydrogen can rediffuse from the steel, so which ductility is restored if cracking does not occur and the environmental conditions are changed so which no hydrogen is produced on the surface of the metal.