Reaction:
Alcohols can be produced from alkenes through reaction with diborane (B2H6 or BH3), followed via treatment with hydrogen peroxide as shown in figure.
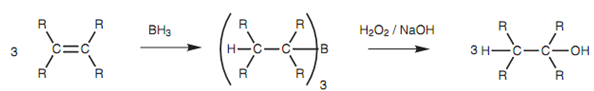
Figure: Hydroboration of an alkene.
The first part of the reaction includes the splitting of a B-H bond in BH3 along with the hydrogen joining one end of the alkene and the boron joining the other. Each of the B-H bonds is split in this manner like that each BH3 molecule reacts with three alkenes to provide an organoborane intermediate in which boron is linked to three alkyl groups. After that this can be oxidized with alkaline hydrogen peroxide to generate the alcohol.