Mechanism:
The mechanism that is displayed in the figure involves the alkene π bond interacting with the empty p orbital of boron to form a π complex. After that one of BH3's hydrogen atoms is transferred to one end of the alkene as boron itself forms an σ bond to another end.
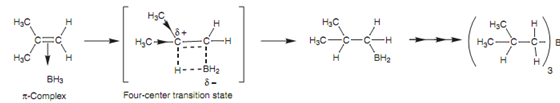
Figure: Mechanism of Hydroboration.
This occurs by a four-centered transition state in which the alkene's π bond and the B-H bond are partially broken, and the eventual C-H and C-B bonds are partially created. There is an imbalance of electrons in the transition state that results in the boron being slightly negative and one of the alkene carbons is being slightly positive. The carbon best capable to handle this will be the most substituted carbon and thus the boron will end up on the least substituted carbon. (Note: boron comprises six valence electrons and is electrophilic. Hence, the addition of boron to the least substituted position in fact follows Markovnikov's rule.)
Because subsequent oxidation with hydrogen peroxide changes the boron with a hydroxyl group, the eventual alcohol will be on the least substituted carbon. Additionally, the addition of the boron and hydrogen atoms occurs such that they are on similar side of the alkene. This is termed as syn-addition.
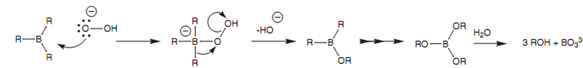
Figure: Mechanism of oxidation with hydroperoxide.